反相液相色谱(RPLC)因具有良好的选择性,已成为当今应用最为广泛的色谱分离模式。然而,在分析一些离解性强的化合物时,单一的RPLC体系并不能得到满意的分离效果[1]。离子对反相液相色谱法(IP-RPLC)通过在流动相中添加离子对试剂,增强带相反电荷溶质的保留从而改善分离,主要用于强离解化合物的分离分析[2,3,4,5,6,7]。在IP-RPLC的应用研究中,科研工作者对影响化合物保留行为的因素一直颇为关注,开展了较为广泛的研究。Fletouris等[8]采用烷基季铵盐作为离子对试剂,对多种青霉素进行IP-RPLC分析,研究了配对离子链长度、流动相pH和浓度,以及柱温对青霉素保留行为的影响。Lu等[9]利用IP-RPLC梯度洗脱研究了氯雷他定及其8种相关化合物的保留行为与流动相pH以及离子对试剂浓度之间的关系。刘文霞等[1]将十二烷基硫酸钠(SDS)作为流动相添加剂,考察了不同色谱条件对表阿霉素及其6种相关物质保留行为的影响,为表阿霉素及其相关物质的分离测定提供了新思路。Burmaoglu等[5]采用蒸发光散射检测器,分别考察了离子对试剂浓度、有机调节剂类型、流动相pH、色谱柱类型、柱温等对唑来膦酸及其相关杂质保留行为的影响,建立了最佳分离分析策略。很显然,在IP-RPLC的应用研究中,人们主要关注离子对试剂的浓度及对离子(counter ion)类型、缓冲溶液的浓度及pH、柱温等对保留的影响,而离子对试剂中的非对离子(non-counter ion)以及缓冲盐类型对溶质保留行为的影响研究相对较少。
k为溶质的保留因子,φ是流动相中有机调节剂的体积分数,S是线性回归得到的常数。
本文以14种磺酸化合物为研究对象,在硅胶基质C18柱上,以甲醇为有机调节剂,采用IP-RPLC进行保留行为研究。首先,固定流动相中离子对试剂为四丁基溴化铵不变,考察缓冲盐类型(磷酸二氢铵、氯化铵和乙酸铵)对磺酸化合物保留行为的影响;然后,固定流动相中缓冲盐为磷酸二氢铵不变,考察四丁基季铵盐离子对试剂(四丁基溴化铵、四丁基磷酸二氢铵、四丁基硫酸氢铵、四丁基硝酸铵和四丁基乙酸铵)中阴离子对磺酸化合物保留行为的影响,并探索了IP-RPLC的保留机制。最后,对log kw、S、CHI与log D的相关性进行了比较。
1 实验部分
1.1 仪器、试剂与材料
实验所用高效液相色谱仪为Waters Alliance 2695(Waters,美国),仪器配有真空脱气机、数码四元泵和120位自动进样器。数据的采集和处理均在Waters Empower色谱管理系统中进行。用996紫外-可见二极管阵列(PDA)检测器在每个化合物的最佳吸收波长处检测其吸收峰。流动相pH值使用SevenMulti型pH/电导率/离子综合测试仪(Metter-Toledo,瑞士)测量。
实验中所用甲醇(HPLC级)购自美国Honeywell公司,所用水均为饮用纯净水(杭州娃哈哈集团);分析纯磷酸二氢铵、磷酸二氢钾、磷酸二氢钠、乙酸铵、氯化铵、氨水(25%~28%)、磷酸(85%)、冰乙酸(98%)和盐酸(36%~38%)均购自南京化学试剂股份有限公司;四丁基溴化铵(99%)购自百灵威公司(上海),四丁基硫酸铵(99%)购自安耐吉化学(上海),四丁基乙酸铵(98%)和四丁基磷酸氢铵(99%)购自毕得医药科技有限公司(上海),四丁基硝酸铵(99%)购自艾览化工科技有限公司(上海)。
本实验中对14种磺酸类化合物进行研究,具体信息见表1。其中,log D7.0值(pH=7.0条件下的log D值)是由本课题组前期工作中利用IP-RPLC测试得到的实验值[16], pKa值由ACD/Labs软件计算得到,溶质静电荷ne、氢键酸性参数A、氢键碱性参数B、极性表面积PSA由
表1 化合物的log D7.0、pKa、ne、A、B和PSA值
Table 1
| No. | Compound | log D7.0 | pKa | ne | A | B | PSA/nm2 |
|---|---|---|---|---|---|---|---|
| SA1 | benzenesulfonic acid | -2.16 | -0.60±0.50 | -1.00 | 0.31 | 0.88 | 0.628 |
| SA2 | 1,5-naphthalenedisulfonic acid | -4.69 | -0.60±0.40 | -2.00 | 0.63 | 1.71 | 1.260 |
| SA3 | 4-chlorobenzenesulfonic acid | -1.08 | -0.83±0.50 | -1.00 | 0.31 | 0.87 | 0.628 |
| SA4 | 4-methylbenzenesulfonic acid | -1.34 | -0.43±0.50 | -1.00 | 0.31 | 0.88 | 0.628 |
| SA5 | 5-amino-2-nanphthalenesulfonic acid | -1.81 | -0.23±0.40 | -1.00 | 0.54 | 1.26 | 0.888 |
| SA6 | 2-amino-1,4-benzenedisulfonic acid | -5.55 | -1.15±0.50 | -2.00 | 0.85 | 1.90 | 1.520 |
| SA7 | 1-naphthalenesulfonic acid | -0.74 | 0.17±0.10 | -1.00 | 0.31 | 0.94 | 0.628 |
| SA8 | 2-naphthalenesulfonic acid | -0.75 | 0.27±0.10 | -1.00 | 0.31 | 0.94 | 0.628 |
| SA9 | 2,4-dimethylbenzenesulfonic acid | -1.09 | -0.36±0.50 | -1.00 | 0.31 | 0.89 | 0.628 |
| SA10 | 4-sulfobenzoic acid | -4.70 | -1.01±0.50 | -2.00 | 0.88 | 1.21 | 1.000 |
| SA11 | 3,5-dichloro-2-hydroxybenzenesulfonic acid | -0.33 | -1.29±0.45 | -1.14 | 0.81 | 0.90 | 0.830 |
| SA12 | 3,5-dicarbomethoxybenzenesulfonic acid | -1.53 | -1.34±0.30 | -1.00 | 0.31 | 1.55 | 1.550 |
| SA13 | 4-hydroxybenzenesulfonic acid | -2.70 | -0.23±0.50 | -1.01 | 0.81 | 1.15 | 0.830 |
| SA14 | 3-sulfobenzoic acid | -4.36 | -0.99±0.15 | -2.00 | 0.88 | 1.21 | 1.000 |
log D7.0 (log D under pH 7.0) was obtained from our previous work[
1.2 色谱条件
色谱柱:Welch Ultimate® XB-C18(150 mm×4.6 mm, 5 μm,月旭科技(上海)股份有限公司);柱温:30 ℃;流速:1.0 mL/min;进样量:5 μL。各化合物的进样质量浓度均为50 mg/L,所有样品的保留时间(tR)均为至少3次独立进样的平均值。
流动相①: (甲醇+10 mmol/L四丁基溴化铵)-(20 mmol/L缓冲盐+10 mmol/L四丁基溴化铵,pH 7.0),分别使用磷酸二氢铵、氯化铵和乙酸铵作为缓冲盐。
流动相②: (甲醇+10 mmol/L四丁基季铵盐)-(20 mmol/L磷酸二氢铵+10 mmol/L四丁基季铵盐,pH 7.0),分别使用含不同阴离子的5种四丁基季铵盐(四丁基溴化铵、四丁基磷酸二氢铵、四丁基硫酸氢铵、四丁基硝酸铵和四丁基乙酸铵)作为离子对试剂。
1.3 实验方法
采用尿嘧啶测定死时间t0,所有化合物均使用等度洗脱,根据化合物疏水性差异,每个化合物至少在4个不同的甲醇体积百分数(70%~10%,间隔5%~10%)下测定tR,使用双点校正法(DP-RTC)校正[17]。根据k=(tR-t0)/tR计算保留因子k,根据方程(1)建立log k-φ方程,求得kw和S值,根据方程(2)计算CHI值。采用Origin 9.4进行相关模型建立及数据分析。
2 结果与讨论
2.1 缓冲盐类型对磺酸化合物保留行为的影响
由于磺酸化合物具有较小的pKa值(见表1),在实验环境下(pH=7.0)带有完全的负电荷,为典型的强离解化合物,并且磺酸化合物具有明显的紫外特征吸收,可用使用最为普遍的二极管阵列检测器进行检测,因此,我们选择磺酸化合物作为模型化合物。在分析强离解酸性化合物时,四丁基季铵盐为最常使用的离子对试剂,在pH=7.0的条件下,四丁基季铵盐带正电荷,与磺酸化合物形成离子对。实验中采用甲醇作为有机调节剂,固定流动相中离子对试剂为四丁基溴化铵不变,分别以磷酸二氢铵、氯化铵和乙酸铵作为缓冲盐(浓度相同,pH相同),考察缓冲盐类型对磺酸化合物保留行为的影响。
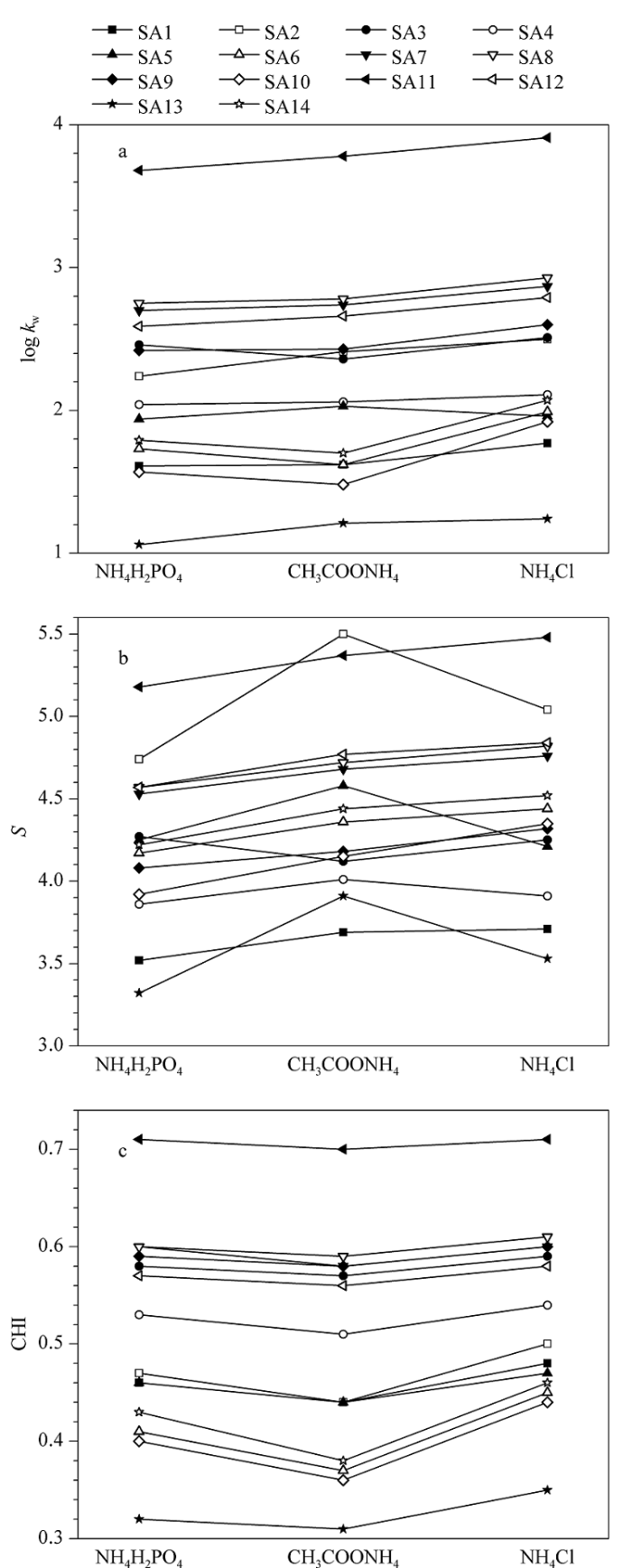
在不同的甲醇比例下,分别获取3种缓冲盐条件下磺酸化合物的k值,并建立log k-φ模型(方程(1)),结果发现,模型的线性相关系数R2均大于0.99。由log k-φ模型获取每个化合物在不同流动相下的log kw和S值,并根据方程(2)计算各化合物的CHI值。3种缓冲盐体系下磺酸化合物的log kw、S和CHI值如图1所示。从图1a可以看出,氯化铵体系下溶质的log kw值最大,而大部分溶质在磷酸二氢铵体系下的log kw值最小,表明流动相中氯离子的存在有利于增强磺酸化合物的保留。图1b显示大部分溶质在氯化铵体系下的S值最大,但SA2、SA4、SA5和SA13在乙酸铵体系下的S值明显大于其他两种缓冲盐体系。图1c显示各化合物的色谱疏水指数CHI在乙酸铵体系下最小。尽管在3种缓冲盐体系下的CHI值存在一定差别,但总体上同一化合物在各体系下对应的CHI值近似相等(见图1c)。
图1
图1
不同缓冲盐体系下磺酸化合物的(a) log kw、(b) S以及(c) CHI
Fig. 1
(a) log kw (logarithm of retention factors of solutes when 100% aqueous phases were used as the mobile phase), (b) S (intercept of the linear solvent strength model) and (c) CHI (chromatographic hydrophobic index) values for sulfonic acid compounds with different buffer solutions
2.2 离子对试剂非对离子对磺酸化合物保留行为的影响
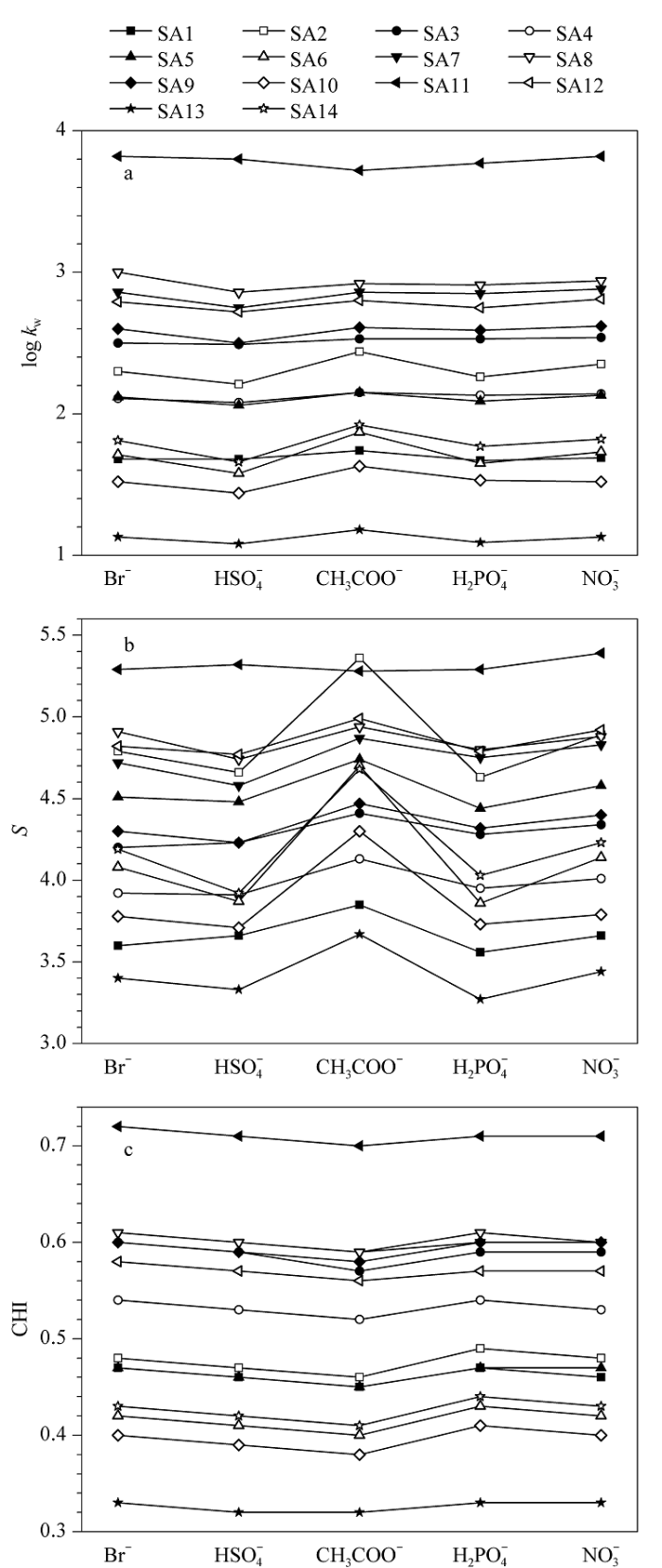
以甲醇作为有机调节剂,固定流动相中磷酸二氢铵作为缓冲盐不变,分别采用四丁基溴化铵、四丁基磷酸二氢铵、四丁基硫酸氢铵、四丁基硝酸铵和四丁基乙酸铵作为离子对试剂,考察离子对试剂非对离子对磺酸化合物保留行为的影响。获取5种流动相下化合物的k值,建立log k-φ线性方程,相关系数R2仍然大于0.99。由log k-φ线性方程得到各磺酸化合物的log kw和S值,进一步根据方程(2)计算相应的CHI值。使用不同阴离子四丁基季铵盐时,各化合物的log kw、S和CHI值如图2所示。从图2a可以看出,SA8和SA11在Br-作为季铵盐阴离子时的log kw最大,SA3、SA7、SA9和SA12在
图2
图2
离子对试剂不同阴离子时磺酸化合物的(a) log kw、 (b) S以及(c) CHI
Fig. 2
(a) log kw, (b) S and (c) CHI values for sulfonic acid compounds with different ion-pair reagents
Br-,
我们的前期研究[18]证明,核酸样品的保留在微小的流动相强度变化下产生的巨大变化,与其非常大的S值相关。因此,相对于四丁基溴化铵、四丁基磷酸二氢铵、四丁基硫酸氢铵和四丁基硝酸铵,磺酸化合物在四丁基乙酸铵体系下对流动相有机调节剂强度的变化更为敏感。有趣的是,所有化合物的CHI仍然是在CH3COO-的存在下最小,与缓冲盐类型对磺酸化合物CHI的影响一致。但从CHI数值对比来看,14种磺酸化合物在5种不同的离子对试剂下得到的CHI值相差不大(最大误差值为0.03)。因此,可认为CHI近似相等(见图2c)。以上结果表明,离子对试剂中的阴离子也会影响磺酸化合物的保留行为,对log kw的影响较为复杂,对S值的影响具有一定的共性,对CHI的影响不大。
2.3 IP-RPLC的保留机理
在动态离子交换模式保留机理中,疏水性的四丁基铵盐可以吸附在固定相表面,形成双电层,磺酸化合物通过与阴离子的交换实现保留,保留主要取决于磺酸化合物所带阴离子电荷的数量。然而,我们的实验表明,具有2个净电荷(ne=-2)的化合物SA2、SA6、SA10的保留(log kw)甚至比相同色谱条件下只有1个净电荷(ne=-1)的化合物(SA7、SA12等)的保留弱(见图1a和2a)。相反,保留最强的化合物为SA11,对应净电荷为-1.14,具有最大的log D值(-0.33),即疏水性最强。因此,动态离子交换模式保留机理占主导地位不成立,猜测离子对模式保留机理占主导地位。
在离子对模式保留机理中, 磺酸化合物的保留与和对离子(四丁基铵盐)的结合力以及所形成中性化合物的疏水性有关。以四丁基溴化铵和磷酸二氢铵条件下的保留为例,磺酸化合物log kw的顺序为SA11>SA8>SA7>SA12>SA3>SA9>SA2>SA4>SA5>SA14>SA6>SA1>
SA10>SA13(见图1a),呈现出与磺酸化合物的疏水性(log D)、与四丁基铵离子的静电结合能力、空间位阻等多重因素相关的保留。有研究[21]表明,在IP-RPLC分析时向流动相中加入与对离子相同电荷的离子会引起溶质保留的变化。Jones等[21]在分离不同的硫酸乙酰肝素二糖时, 利用二糖的负电荷与烷基铵离子对试剂的正电荷发生静电相互作用实现保留,通过在流动相中添加2.5 mmol/L铵离子提高了结构相近的硫酸乙酰肝素二糖IIS和IIIS的分离度,并提出丁胺与铵离子之间的竞争是实现这两种异构体分离的主要原因;该研究充分说明,不同的阳离子可以竞争溶质阴离子,从而导致溶质保留的差异。我们的实验中,在不同的阴离子添加剂条件下,非目标阴离子(
2.4 log kw、S、CHI与log D相关性的比较
在IP-RPLC模型下,电荷作用和氢键作用均会影响离解化合物的保留,可以在log D-log kw模型中引入溶质静电荷ne、氢键酸碱性参数A和B,建立QSRR模型[14],用于log D或保留的预测。在磷酸二氢铵、氯化铵和乙酸铵3种缓冲盐体系下,我们分别以14种磺酸化合物的log D7.0、log kw、ne、A和B进行多元线性拟合,建立QSRR模型,结果如表2所示。可以看出,3种流动相条件下的log D7.0和log kw之间均有良好的线性相关性,R2达到0.98。为了进一步改善方程的相关性,我们将溶质的极性表面积PSA引入到上述方程中,发现方程的R2进一步增大,线性得到改善。同样,在引入参数ne、A和B的情况下,以S对log D7.0作线性方程,可以得到良好的线性相关性,当引入PSA后,氯化铵体系下的线性相关性得到明显改善,线性相关系数R2由0.96增大到0.99(见表2)。最后,分别对3种缓冲盐体系下得到的CHI对log D7.0进行线性拟合,结果显示,PSA引入前后log D7.0-CHI模型的线性相关性R2均在0.98以上。表2中的结果表明,溶质的极性表面积PSA会影响溶质的保留,PSA的加入有利于log D7.0-log kw、log D7.0-S和log D7.0-CHI模型的改善。
表2 不同缓冲盐体系下磺酸化合物的log kw、S、CHI与logD之间的线性关系
Table 2
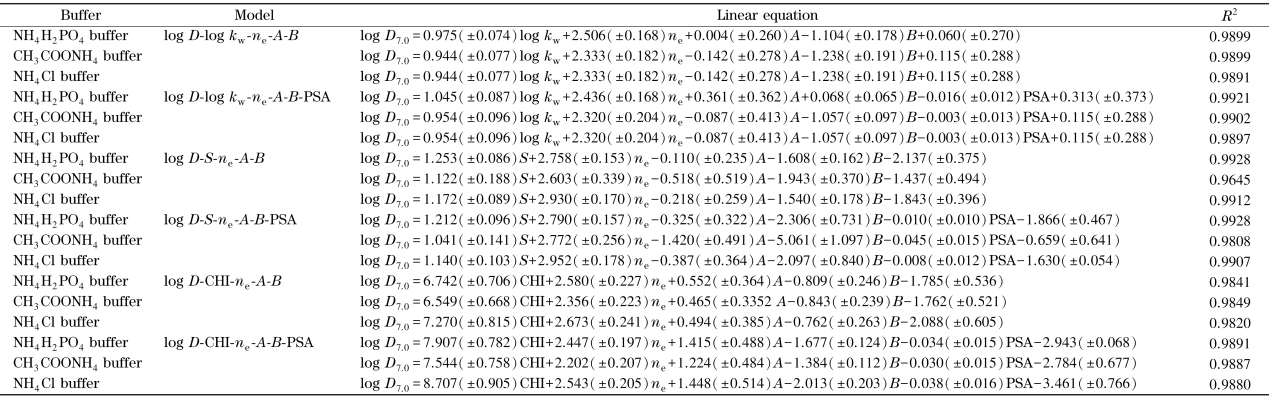 |
引入溶质静电荷ne、氢键酸碱性参数A和B、极性表面积PSA,分别用14种磺酸化合物的logD7.0值与在5种不同离子对试剂条件下得到的log kw、S以及CHI值,进行多元线性拟合,得到如表3所示的线性方程。可以看出,log D7.0和log kw、S以及CHI之间均具有良好的线性关系,相关系数R2均在0.98以上。
表3 不同离子对试剂体系下磺酸化合物的log kw、S、CHI与log D.o之间的线性关系
Table 3
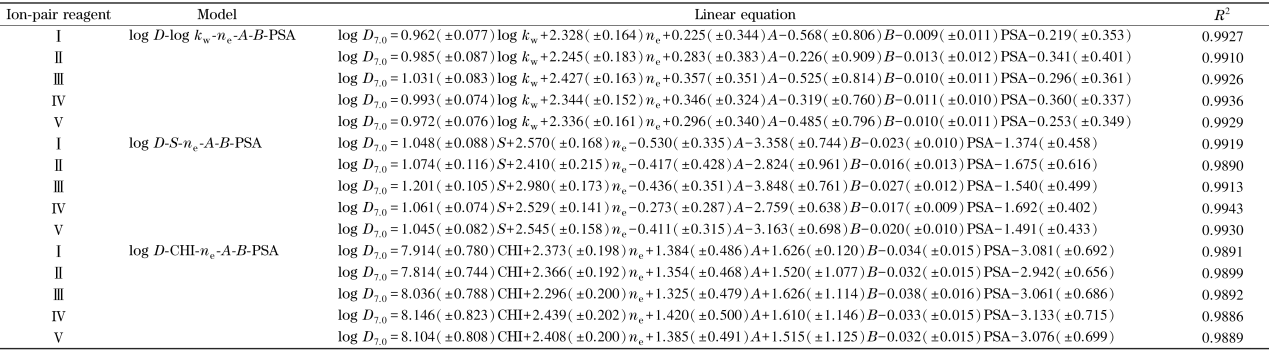 |
I: tetrabutylammonium bromide; I: tetrabutylammonium hydrogen sulfate; I: tetrabutyl ammonium acetate; IV : tetrabutylammonium hydrogen phosphate; V : tetrabutylammonium nitrate.
用IP-RPLC研究log D相关的QSRR模型,通常以log kw作为疏水性指数,建立log D和log kw之间的线性关系。我们的研究发现,log D与S以及CHI之间也具有良好的线性相关性。强离解酸性化合物进行IP-RPLC分析时,当流动相中使用不同的缓冲盐阴离子或者离子对试剂阴离子,即使得到的log kw和S值存在一定差异,但最终获取的色谱疏水指数CHI几乎相等。因此,相比log kw和S而言,CHI更适用于QSRR模型的建立。
3 结论
本研究发现IP-RPLC中缓冲盐阴离子以及离子对试剂阴离子均会影响磺酸化合物的保留行为。相同离子对试剂情况下,氯化铵体系下的log kw最大;相同缓冲盐的情况下,离子对试剂中弱离解性阴离子(乙酸根)的存在有利于增加磺酸化合物的S值。我们推测磺酸化合物的IP-RPLC保留机理同时存在着离子对模式和动态离子交换模式,并以离子对模式为主。对于强离解酸性化合物,由于在IP-RPLC不同缓冲盐和不同非对离子条件下获得的log kw和S值存在着一定的差异,而CHI值相对稳定,因此CHI更适用于QSRR模型的建立。
参考文献
A method has been developed and validated for analysis of zoledronic acid (ZOL) and its related substances by ion-pair reversed-phase high performance liquid chromatography with evaporative light scattering detection (ELSD). Chromatographic separation was achieved with gradient elution by using a C18 column, mobile phase containing 12 mM ammonium acetate buffer and 35 mM n-pentylamine, whose pH value is 7.0, and 5% acetonitrile. The mobile-phase flow rate was 1.0 mL/min. The calibration plot was linear in the range from 0.4 mg/mL to 6.0 mg/mL for ZOL and from 6.25 μg/mL to 100 μg/mL for its related substances. ZOL and its related substances, namely imidazole-1-yl-acetic acid, phosphate, phosphite and degradation products did not interfere with each other. The method was rapid, linear, accurate and reproducible. The high performance liquid chromatographic method that has been developed to determine the related substances and assay of ZOL can be used simultaneously to evaluate the quality of regular samples. It can be also used to test the stability samples of ZOL.
Reversed-phase liquid chromatography (RPLC) based octanol-water partition coefficient (logP) or distribution coefficient (logD) determination methods were revisited and assessed comprehensively. Classic isocratic and some gradient RPLC methods were conducted and evaluated for neutral, weak acid and basic compounds. Different lipophilicity indexes in logP or logD determination were discussed in detail, including the retention factor logk corresponding to neat water as mobile phase extrapolated via linear solvent strength (LSS) model from isocratic runs and calculated with software from gradient runs, the chromatographic hydrophobicity index (CHI), apparent gradient capacity factor (k') and gradient retention time (t). Among the lipophilicity indexes discussed, logk from whether isocratic or gradient elution methods best correlated with logP or logD. Therefore logk is recommended as the preferred lipophilicity index for logP or logD determination. logk easily calculated from methanol gradient runs might be the main candidate to replace logk calculated from classic isocratic run as the ideal lipophilicity index. These revisited RPLC methods were not applicable for strongly ionized compounds that are hardly ion-suppressed. A previously reported imperfect ion-pair RPLC method was attempted and further explored for studying distribution coefficients (logD) of sulfonic acids that totally ionized in the mobile phase. Notably, experimental logD values of sulfonic acids were given for the first time. The IP-RPLC method provided a distinct way to explore logD values of ionized compounds.Copyright © 2017 Elsevier B.V. All rights reserved.
n-Octanol/water partition coefficients (logKow) for persistent organic pollutants (POPs) including polycyclic aromatic hydrocarbons (PAHs), polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and decabromodiphenylethane (DBDPE) have been determined by a modified method of reversed-phase high performance liquid chromatography (RP-HPLC). A dual-point retention time correction (DP-RTC) was used to rectify chromatographic retention time (tR) shift resulted from stationary phase aging and so on. Based on this correction, the relationship model between logKow and logk(w), the logarithm of the retention factor extrapolated to pure water, was trained by a set of model compounds (a total of 37) with reliable experimental logKow as training set, including benzene homologues, PAHs and PCDD/Fs-related compounds. A linear regression equation of logKow = (1.18 +/- 0.02) logk(w) + (0.36 +/- 0.11) was established with correlation coefficient (R2) of 0.985, cross-validated correlation coefficient (R2cv) of 0.983 and standard deviation (SD) of 0.16. This quantitative structure retention relationship (QSRR) model was further validated using four verification compounds, biphenyl, fluorene, PCDD 1 and PCDF 114, with reliable experimental logKow val- ues. The RP-HPLC-determined Kow values showed good consistency with shake-flask (SFM) or slow-stirring (SSM) results, especially for highly hydrophobic compounds. Then, the logKow values for 29 POPs of wide interest were evaluated by the improved RP-HPLC method for the first time. The DP-RTC-HPLC method is recommended for the determination of the logKow values of POPs with strong hydrophobicity.
Reversed-phase liquid chromatography (RPLC) based octanol-water partition coefficient (logP) or distribution coefficient (logD) determination methods were revisited and assessed comprehensively. Classic isocratic and some gradient RPLC methods were conducted and evaluated for neutral, weak acid and basic compounds. Different lipophilicity indexes in logP or logD determination were discussed in detail, including the retention factor logk corresponding to neat water as mobile phase extrapolated via linear solvent strength (LSS) model from isocratic runs and calculated with software from gradient runs, the chromatographic hydrophobicity index (CHI), apparent gradient capacity factor (k') and gradient retention time (t). Among the lipophilicity indexes discussed, logk from whether isocratic or gradient elution methods best correlated with logP or logD. Therefore logk is recommended as the preferred lipophilicity index for logP or logD determination. logk easily calculated from methanol gradient runs might be the main candidate to replace logk calculated from classic isocratic run as the ideal lipophilicity index. These revisited RPLC methods were not applicable for strongly ionized compounds that are hardly ion-suppressed. A previously reported imperfect ion-pair RPLC method was attempted and further explored for studying distribution coefficients (logD) of sulfonic acids that totally ionized in the mobile phase. Notably, experimental logD values of sulfonic acids were given for the first time. The IP-RPLC method provided a distinct way to explore logD values of ionized compounds.Copyright © 2017 Elsevier B.V. All rights reserved.